The drug Cantil® was introduced in the early 1960s as a new anticholinergic drug for gastrointestinal disorders, including irritable bowel syndrome (IBS) and functional colopathy, as represented. The active principle of Cantil was N-methyl-3-piperidol benzilate bromo-methylate, better known as mepenzolate bromide. The drug was efficient to relieve abdominal pain, abnormal bowel action, such as diarrhea, and gaseous distress. It worked by reducing spontaneous bowel contractions, lowering the release of pepsin and decreasing stomach acid production. It was used notably to treat peptic ulcers of the stomach and inflammation of the intestine.
In recent years, the drug has been shown to function as a muscarinic receptor antagonist with bronchodilatory and anti-inflammatory activities. The discovery renewed interest for this compound, which showed beneficial effects in animal models of chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis. Mepenzolate bromide acts as an antagonist for human muscarinic M3 receptor (hM3R) and this binding account for the anti-inflammatory and bronchodilatory activities. There are two enantiomers, (R/S)-mepenzolate, with a similar anti-inflammatory activity. But the (R) enantiomer showed a higher affinity for hM3R than the (S) enantiomer. In vivo, the bronchodilatory activity of (R)-mepenzolate was superior to that of (S)-mepenzolate. Mepenzolate bromide displays other activities. Notably, it is a blocker of microglial-expressed G-protein coupled receptor 109A (GPR109A, also known as the niacin receptor or the hydroxycarboxylic acid receptor 2 (HCA2)). Cantil® is no longer used today for the treatment of gastrointestinal disorders.

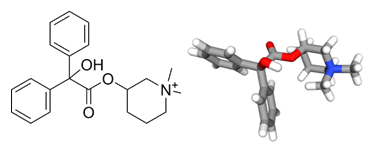
mepenzolate