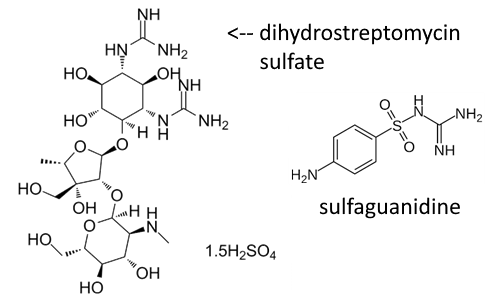
Entercine® was used to treat acute diarrhea and other severe intestinal disorders, notably in the frame of dietary intoxication. The oral drug (capsule) contained the aminoglycoside antibiotic dihydrostreptomycin sulfate in association with the sulfonamide antibiotic sulfaguanidine. It contained two additional active ingredients: (i) the metal-complexing hydroxyquinoline derivative dibromoxyquinoleine (or 5,7-dibromo-8-hydroxyquinoline), an intestinal antiseptic active against certain bacteria and amoeba and (ii) the anticholinergic agent homatropine methylbromide, known to inhibit muscarinic acetylcholine receptors. The combination of these active principles provided a robust antiseptic action. Like streptomycin, dihydrostreptomycin sulfate is among the most common aminoglycoside antibiotics used in human and veterinary medicine. It is a potent inhibitor of protein translation, acting on bacterial ribosomes via a direct binding to the mechanosensitive channel of large conductance channel protein (MscL). Upon binding to MscL, the drug modifies the protein conformation to allow the passage of potassium ions and glutamate out of, and dihydrostreptomycin into, the cell. An efficient gating mechanism at the origin of the antiseptic action. Entercine® is not on the market anymore but dihydrostreptomycin sulfate remains largely used in other preparations.