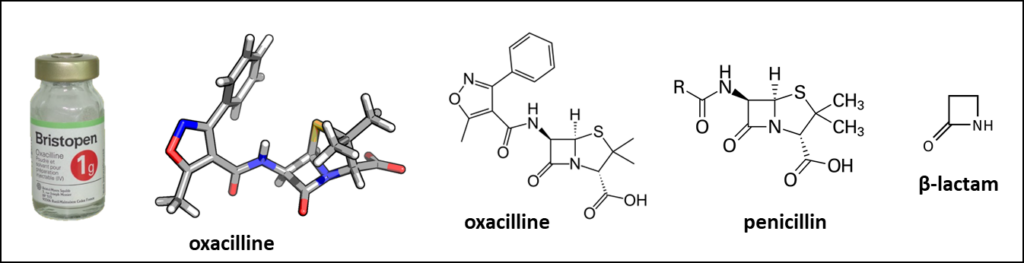
The active ingredient of Bristopen® is the penicillin (β-lactam) antibiotic oxacillin, introduced in the clinic in the early 1960s for the treatment of bacterial infections, notably to treat resistant Staphylococcal infections. Both oral and parenteral formulations of Bristopen were proposed. The product was partially resistant to gastric acid degradation, it could be administered orally.

Oxacillin is a hemisynthetic product obtained by reacting 5-methyl-3-phenyl-4-isoxazolcarboxylic acid chloride with the industrial intermediate 6-aminopenicillanic acid (6-APA). This latter product is produced on a large scale by enzymatic cleavage of penicillins G or V side chain with a penicillin amidase. Like other beta-lactam antibiotics, oxacillin interferes with penicillin-binding proteins (PBPs) normally required for bacterial cell wall formation. After binding to PBPs, the bacterial cell wall weakens or undergoes lysis in the presence of the antibiotic. Oxacillin is a second generation penicillin [(5-methyl-3 phenyl-4-isoxazolyl) penicillin]. For about sixty years now, oxacillin is used to treat moderate-to-serious infections with susceptible penicillinase-producing Staphylococci. It remains one of the most important penicillin antibiotics used worldwide, as a first-line agent. It is traditionally used for the treatment of methicillin-susceptible Staphylococcus aureus infections.
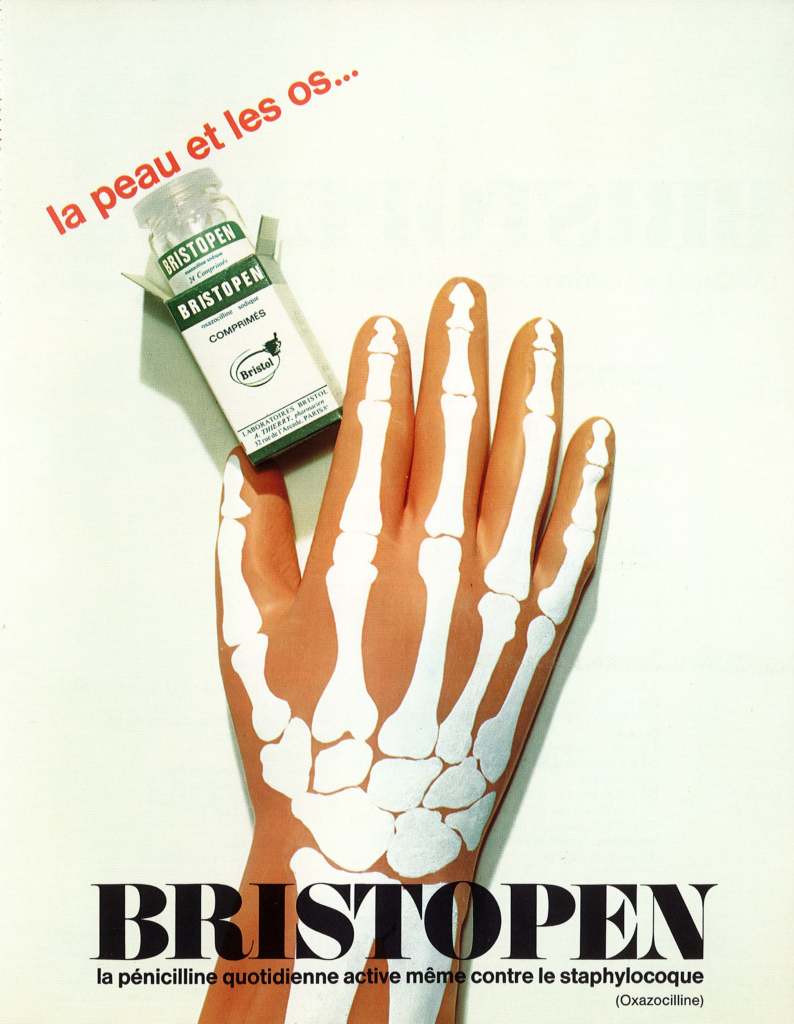
The advertising made reference to the use of Bristopen® to treat skin, bone, or hand infections, following bone surgery, open fracture, or burns for examples.