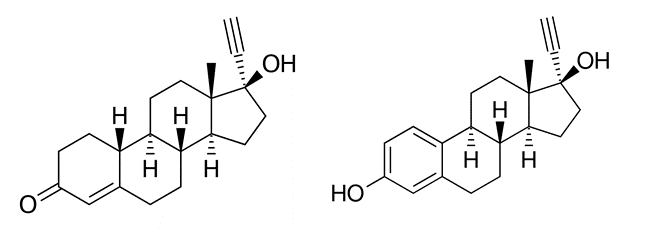
An oestro-progestative pill to put to rest the ovary. This was the promotional comment to support the two products anovlar (4mg) and milli-anovlar (1 mg). Both products contained norethisterone acetate and the estradiol derivative ethinyl estradiol, a combination of oral contraceptive products still used today. Norethisterone is a synthetic progestin orally administered with strong endometrial activity. It can be metabolically converted into ethinyl-estradiol which is a synthetic form of estrogen. Anovlar (4 mg of norethisterone acetate plus 0.05 mg of ethinyloestradiol) has been extensively used in the 1960-70s as a cyclical oral contraceptive pill. The product was well-tolerated; side-effects were moderate (nausea, increase in weight) and the cycle control was considered “remarkably good”. In the early 1960s, the introduction of oral contraceptive drugs was a true revolution, allowing women to effectively regulate their own fertility for the first time in human history.


In 2017, a stamp was published in Belgium to honor the Belgian doctor Ferdinand Peeters who, in 1959-1960, developed Anovlar as the first clinically applicable contraceptive pill. Dr Peeters, a practising gynecologist from Turnhout (near Antwerp, Belgium) had a pivotal role in the development of a practical and viable modality of oral contraception. He discovered the potency and safety of the combination of norethisterone acetate and ethinylestradiol for ovulation suppression, and proposed his discovery to Schering AG in Berlin (Germany). This pill set the standard for all future pills to follow.