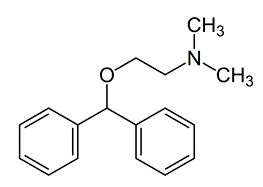
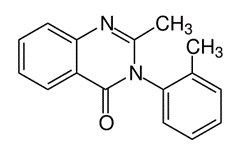
The drug Mandrax® was initially prescribed for the treatment of insomnia in overactive, nervous, and/or anxious people. The quality of the sleep induced by Mandrax was close to that of natural sleep (a more natural sleep than with barbiturates). Each oral tablet contained two active principles. On the one hand, the quinazolinone derivative methaqualone (250 mg) which provided a sedative action, via a depressive effect on the central nervous system. On the other hand, the antihistaminic agent diphenhydramine hydrochloride (25 mg) used to help the patient relaxing and falling asleep. Diphenhydramine has been shown to potentiate the sedative properties of methaqualone.



Unfortunately, several cases of poisoning with Mandrax have been reported, causing severe peripheral motor and sensory neuropathy. Mandrax overdose was shown to induce schizophrenia-like psychosis. In Europe, the drug was declared illegal and toxic in the lates 1970s. But its use continued in some other countries, such as South Africa where many cases of Mandrax abuses have been reported (often combined with other illicit and dangerous products). Methaqualone even represented a substitute for heroin in some cases. It has been shown that users could develop a tolerance and dependence to methaqualone after just a few days of use. The drug ceased being manufactured in the mid 1980s. Unfortunately, methaqualone remains used illegally today in some places. It is a CNS depressant, hypnotic compound that may cause neurotoxicity in overdose. Not a drug to recommend at all.