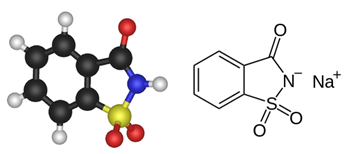
The acronym ODA stands for Obesity-Diabetes-Atherosclerosis. ODA was one of the first product proposed to replace a conventional sugar cube (made of saccharose, also called sucrose) with an unsweetened equivalent. It was proposed to patients suffering from atherosclerosis or diabetes, to accompany their tea or coffee. The product contained saccharin (o-benzoic sulfimide, or 1,1-dioxo-1,2-benzothiazol-3-one) which is a well-known non-nutritive sweetener, used for more than a century as an artificial sweetener.


Saccharin was discovered in 1879 by the Russian-German chemist Constantin Fahlberg, in the laboratory of Prof. Ira Remsen at Johns Hopkins University (Baltimore, US). It was later characterized as a ligand for sweet taste receptors. Saccharin exhibits both sweetening and sweet inhibition activities. It remains one of the most used sugar substitutes, as an alternative to table sugar to control weight and obesity. The crystalline hydrate of saccharin is a hundred times as sweet as sucrose. Saccharin is approved for use in foods in the U.S. and Europe. It is a safe product, if taken in acceptable quantities daily. The acceptable daily intake – ADI – is 15 milligrams per kilogram body weight per day in humans. Saccharin can greatly help to reduce caloric and glycemic intake while providing desired sweetness.
Today, saccharin remains consumed by millions of people worldwide, even if other low-/no-calorie sweeteners are available (like aspartame, acesulfame-K, sucralose, cyclamate, thaumatin and steviol glycosides).