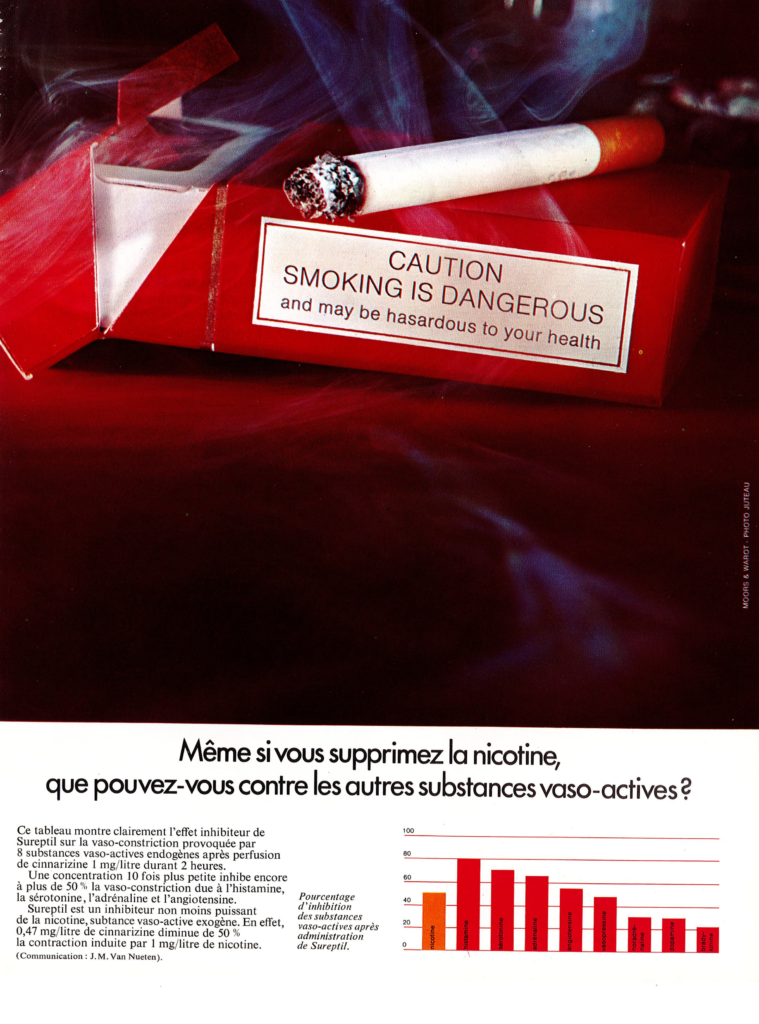
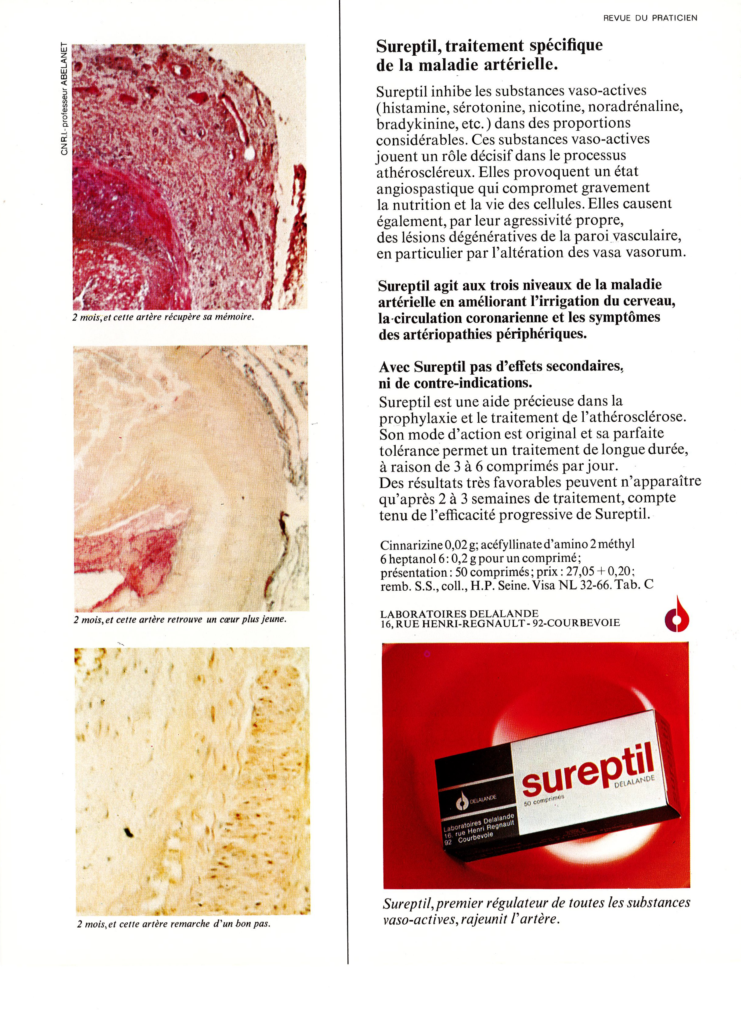
The drug Sureptil® contained two active principles: (i) the piperazine derivative cinnarizine which has antihistaminic, antiserotoninergic, antidopaminergic, and calcium channel-blocking activities, and (ii) the synthetic molecule heptaminol acefyllinate (a theophylline derivative) which is a peripheral vasodilator. The drug combination was used to improve blood circulation and tissue oxygenation. Sureptil® was removed from the market in 2003. The drug was Sureptil® in France, and Stugeron® in other countries, mainly used to control travel sickness.
Cinnarizine is an anti-histaminic agent (antagonist of H1-histamine receptors) but its has also antiserotonergic, antidopaminergic, and calcium channel-blocking activities. This compound has been used for the treatment of nausea and motion sickness, as well as vertigo associated with Ménière’s syndrome, taking advantage of its anti-vasoconstrictor activity and its capacity to reduce blood viscosity. Cinnarizine represents an efficacious and well-tolerated prophylactic antimigraine medication, notably in adults with migraine-associated vertigo such as vestibular migraine. It remains used today in a few countries (not in the US). The treatment benefit of this compound should be balanced with its adverse effects because the long-term use of this compound has been suspected to increase the risk for Parkinsonism, dyskinesia, and dystonia. It has shown extrapyramidal side effects.

Sureptil was used to treat cerebral circulatory insufficiency. The cover image was intended to illustrate the improved brain flow upon treatment with Sureptil, with a type of extended blueish brain, like waves or clouds.