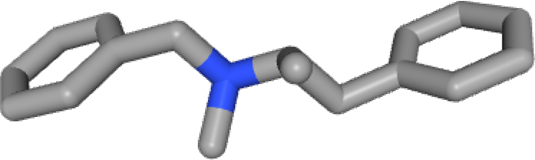
Inapetyl® belonged to a class of drugs used in the management of obesity, a somewhat controversed category of products due to their potential side effects.
The active principle here is benzphetamine hydrochloride, a sympathomimetic product approved for short term treatment of obesity, for a few weeks, usually up to 12 weeks maximum. The drug facilitated weight loss (together with lifestyle intervention, physical activity and diet). It is an inhibitor for reuptake of noradrenalin in the synapse (a noradrenergic drug), approved by the FDA since 1960. It was a controlled substance, classified as “DEA Schedule III” which corresponds to a drug with a moderate to low potential for physical and psychological dependence. The diverse bioactive metabolites of benzphetamine include d-amphetamine and d-methamphetamine which could be used for the medication of cocaine-associated disorders.
Inapetyl® was well prescribed in the 1970-85s, considered as a fast-acting anorexigen, acting primarily on the neurochemical transmitters of the central nervous system to reduce food intake. It could help patients to achieve and maintain weight loss, acting as an ‘appetite suppressant’, hence the evocative name Inapetyl®. It is no longer used today (removed from the market in 1987); there are better therapeutic options available.