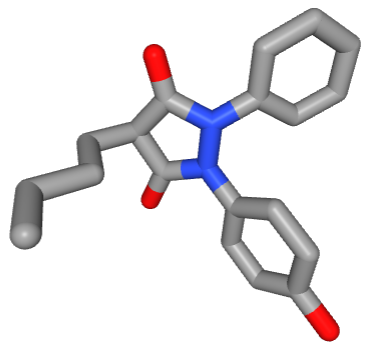
The active ingredient of Tandéril®, known as oxyphenbutazone (OPB or 1-phenyl-2-(p-hydroxyphenyl)-3,5-dioxo-4-n-butyl-pyrazolidine), was a classical, non-expensive, non-steroidal anti-inflammatory drug (NSAID) used for many years to treat a large variety of inflammatory conditions: arthritis, post-operative inflammation, sarcoidosis. It was withdrawn from markets worldwide in mid-1980s due to toxic side effects (bone marrow suppression).
Oxyphenbutazone is also the active metabolite of phenylbutazone, an anti-inflammatory drug which remains used in human and veterinary medicine. The drug has analgesic and antipyretic actions useful to treat acute pain and muscular-skeletal disorders, such as in cases of ankylosing spondylitis and rheumatoid arthritis. But it shows a non-selective action on the enzyme cyclooxygenase 1 (COX-1 isoform), at the origin of adverse reactions such as gastritis, gastric ulcers, venous thrombosis, nephritis and chronic renal injury. For this reason, phenylbutazone was banned from human use, for all but a few diseases, in the early 1980s. But today, phenylbutazone remains a drug widely used in veterinary medicine (in horses notably to treat musculoskeletal disorders), although it is not permitted in the European Union for use in animals destined for the food chain.